Data Collection
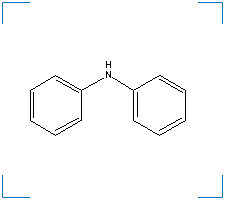
Diphenyl Amine
CAS Number: 122-39-4
Basic Information
 Synonym(s): Anilinobenzene; big dipper; Diphenyl amine; Diphenylamine ; C.I. 10355; DFA; DPA; N,N-diphenylamine; No Scald; N-Phenylbenzenamine; N-Phenyl Aniline; (phenylamino)benzene; Phenylbenzenamine; scaldip
Synonym(s): Anilinobenzene; big dipper; Diphenyl amine; Diphenylamine ; C.I. 10355; DFA; DPA; N,N-diphenylamine; No Scald; N-Phenylbenzenamine; N-Phenyl Aniline; (phenylamino)benzene; Phenylbenzenamine; scaldip
Formula: C12H11N
Molecular Weight: 169.23
Boiling Point (°C): 302
Melting Point (°C): 52
Contaminant Type: Diphenyl Amine is a fungicide.
Notes: Floral odor. White crystalline solid
Solubility (in water)
- Solubility:
40
mg/l
ak
Temperature (°C): 25
- Solubility:
300
mg/l
ak
Temperature (°C): 25
Half-Life (in soil unless otherwise noted)
- t1/2:
1-4
weeks
f
Environment: Scientific judgement based upon estimated unacclimated aqueous aerobic biodegradation half-life
Toxicity Effects
- Organism Type:
Crustaceans
ak
Common Name: Daphnia
Scientific Name: Daphnia magna
Toxicity: 2.3 mg/l
Test: 24h EC50
- Organism Type:
Fish
ak
Common Name: Zebra danio
Scientific Name: Brachydanio rerio
Toxicity: 2.2 mg/l
Test: 48h LC50
- Organism Type:
Fish
ak
Common Name: Orfe
Scientific Name: Idus idus, or Leuciscus idus
Toxicity: 20 mg/l
Test: 48h LC0
- Organism Type:
Fish
ak
Common Name: Japanese medaka, Japanese killfish
Scientific Name: Oryzias latipes
Toxicity: 5.1 mg/l
Test: 48h LC50
- Organism Type:
Mammals
g
Common Name: Guinea pig
Toxicity: 300-1000 mg/kg
Test: LD50
Octanol-Water Partition Coefficients
- Log Kow:
3.5
ak
Formulation: measured
- Log Kow:
3.6
ak
Formulation: calculated
Work Cited
|
f
|
Howard, P. H., R. S. Boethling, W. F. Jarvis, W. M. Meylan, and E. M. Michalenko. 1991. Handbook of Environmental Degradation Rates. Lewis Publishers., Chelsea, MI.
|
|
g
|
Meister, R. T., and C. Sine (Ed). 2003. Crop Protection Handbook, volume 89. Meister Publishing Company, Willoughby, OH.
|
|
ak
|
Verschueren, K. 2001. Handbook of Environmental Data on Organic Chemicals, 4th edition. John Wiley & Sons, Inc., New York.
|