Data Collection
Chlorothalonil
CAS Number: 1897-45-6
Basic Information
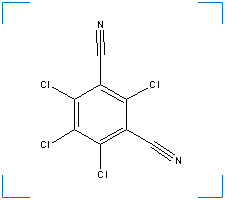 Synonym(s): 1,3-dicyano-2,4,5,6-tetrachlorobenzene; 2,4,5,6-Tetrachloro-1,3-benzenedicarbonitrile; 2,4,5,6-tetrachloro-1,3-dicyanobenzene; 2,4,5,6-tetrachloroisophthalonitrile;
Synonym(s): 1,3-dicyano-2,4,5,6-tetrachlorobenzene; 2,4,5,6-Tetrachloro-1,3-benzenedicarbonitrile; 2,4,5,6-tetrachloro-1,3-dicyanobenzene; 2,4,5,6-tetrachloroisophthalonitrile;
Formula: C8Cl4N2
Molecular Weight: 265.91
Melting Point (°C): 250
Contaminant Type: Chlorothalonil is a fungicide.
Notes: Crystals
Additional Information: Chlorothalonil has high binding and low mobility in silty loam and silty clay loam soils, and has low binding and moderate mobility in sand. In studies conducted in water over ten weeks time, chlorothalonil, at low levels, was generally stable. Chlorothalonil was not found in any of 560 groundwater samples collected from 556 sites.
m
Solubility (in water)
- Solubility:
0.6
mg/l
p
Temperature (°C): 25
- Solubility:
0.6 - 1.2
mg/l
g
Half-Life (in soil unless otherwise noted)
Toxicity Effects
- Organism Type:
Crustaceans
b
Common Name: Crustaceans
Toxicity: 1.2-270 mg/l
Test: 96h LC50
- Organism Type:
Crustaceans
e
Common Name: Daphnia
Scientific Name: Daphnia magna
Toxicity: 4.5 µg/l
Test: 48h LC50
- Organism Type:
Crustaceans
y
Common Name: Daphnia
Scientific Name: Daphnia magna
Toxicity: 68 µg/l
Test: 96h LC50
- Organism Type:
Crustaceans
z
Common Name: Daggerblade grass shrimp
Scientific Name: Palaemonetes pugio
Toxicity: 152.9 µg/l
Test: 96h LC50
- Organism Type:
Crustaceans
y
Common Name: Pink shrimp
Scientific Name: Panaeus duorarum
Toxicity: 154 µg/l
Test: 96h LC50
- Organism Type:
Crustaceans
aa
Scientific Name: Paratya australiensis
Toxicity: 16 µg/l
Test: 96h LC50
- Organism Type:
Fish
b
Common Name: Fish
Toxicity: 30-36 µg/l
Test: 96h LC50
- Organism Type:
Fish
y
Common Name: Sheepshead minnow
Scientific Name: Cyprinodon variegatus
Toxicity: 32.0 µg/l
Test: 96h LC50
- Organism Type:
Fish
e
Common Name: Carp
Scientific Name: Cyprinus carpio
Toxicity: 0.11 mg/l
Test: 48h LC50
- Organism Type:
Fish
m
Common Name: Channel catfish
Scientific Name: Ictalurus punctatus
Toxicity: 0.43 mg/l
Test: LC50
- Organism Type:
Fish
g
Common Name: Bluegill
Scientific Name: Lepomis macrochirus
Toxicity: 62 µg/l
Test: 96h LC50
- Organism Type:
Fish
m
Common Name: Bluegill
Scientific Name: Lepomis macrochirus
Toxicity: 0.3 mg/l
Test: LC50
- Organism Type:
Fish
g
Common Name: Rainbow trout
Scientific Name: Oncorhynchus mykiss
Toxicity: 49 µg/l
Test: 96h LC50
- Organism Type:
Fish
m
Common Name: Rainbow trout
Scientific Name: Oncorhynchus mykiss
Toxicity: 0.25 mg/l
Test: LC50
- Organism Type:
Fish
e
Common Name: Columbia River redband trout
Scientific Name: Salmo gairdneri
Toxicity: 7.6-17 mg/l
Test: 4d LC50
- Organism Type:
Mammals
m
Common Name: Rat
Scientific Name: Muridae (Family)
Toxicity: >10000 mg/kg
Test: LD50
- Organism Type:
Molluscs
b
Common Name: Molluscs
Toxicity: 2.9-26 µg/l
Test: EC50
- Organism Type:
Molluscs
y
Common Name: Eastern oyster
Scientific Name: Crassostrea virginica
Toxicity: 3.6 µg/l
Test: 96h LC50
Soil Organic Carbon/Water Partition Coefficients
- Koc:
Log Koc: 2.76, 3.14
k
Octanol-Water Partition Coefficients
Work Cited
|
a
|
Beyond pesticides fact sheets. Pages retrieved November, 2003 from http://www.beyondpesticides.org. Beyond Pesticides, Washington, DC.
|
|
b
|
AQUIRE (AQUatic toxicity Information REtrieval) database (As part of the ECOTOX Database). Pages retreived February, 2003 from http://www.epa.gov/ecotox/. USEPA, Washington, DC.
|
|
e
|
Verschueren, K. 1996. Handbook of Environmental Data on Organic Chemicals, 3rd edition. John Wiley & Sons, Inc., New York.
|
|
g
|
Meister, R. T., and C. Sine (Ed). 2003. Crop Protection Handbook, volume 89. Meister Publishing Company, Willoughby, OH.
|
|
k
|
Montgomery, J. H. 1993. Agrochemicals Desk Reference: Environmental Data. Lewis Publishers, Boca Raton, FL.
|
|
l
|
The ARS Pesticide Properties Database. Pages retrieved November, 2003 from http://www.arsusda.gov/acsl/ppdb.html. USDA, ARS, Alternate Crops & Systems Lab, Beltsville, MD.
|
|
m
|
The University of Manchester Compound Database. Pages retrieved November 2003 from http://www.man.ac.uk/umec/extra/list.html. The University of Manchester, Manchester, England.
|
|
p
|
Syracuse Research Corporation Databases. Pages retrieved November, 2003 from: http://esc.syrres.com. Syracuse Research Corporation, North Syracuse, NY.
|
|
x
|
Armbrust, K. L. 2001. Chlorothalonil and chlorpyrifos degradation products in golf course leachate. Pest Management Science 57:797-802.
|
|
y
|
USEPA. 1999. Reregistration eligibility decision: chlorothalonil list A case 0097. Office of Pesticide Programs Special Review and Reregistration Division, Washington, DC.
|
|
z
|
Key, P. B., S. L. Meyer, and K. W. Chung. 2003. Lethal and sublethal effects of the fungicide chlorothalonil on three life stages of the grass shrimp, Palaemonetes pugio. J. Environ. Sci. Health B38(5):539-549.
|
|
aa
|
Davies, P. E., L. Cook, and D. Goenarso. 1994. Sublethal responses to pesticides of sevral species of Australian freshwater fish and crustaceans and rainbow trout. Environmental Toxicology and Chemistry 13(8):1341-1354.
|